Turbulence
Module 1 – What is turbulence?
You Try:
- 1. Describe the flow of the water in each image.
- 2. Imagine how a molecule of water would move through each of these rivers, or if we dropped some bits of Styrofoam in;
- the molecules and Styrofoam would be “tracers”. Try making a sketch of the path tracers would take in each river.
We poured cream (died green) down half of a PVC pipe. We dripped green dye into the cream to trace how it was flowing.
Watch where the dye flows in the following videos and sketch the path tracer (a molecule of the dye) might take in each of the following situations.
You may want to watch the video several times and pause it often.
You try:
- 3. Based on what you see the green dye doing, make a sketch of the path a tracer might take down the track.
- 4. Try this for 3 tracers. Do all the paths have the same general shape?
You try:
- 5. Based on what you see the green dye doing, make a sketch of the path a tracer might take down the track. While all the molecules are travelling in a general right-to-left direction, some follow a more wobbly path.
- 6. Try this for 3 tracers. Do all the paths have the same general shape?
- 7. How is the flow at the bottom different than at the top?
Consider the videos and your particle path drawings. As you answer the following questions, make a sketch with multiple arrows showing the direction a molecule is travelling at different times.
You try:
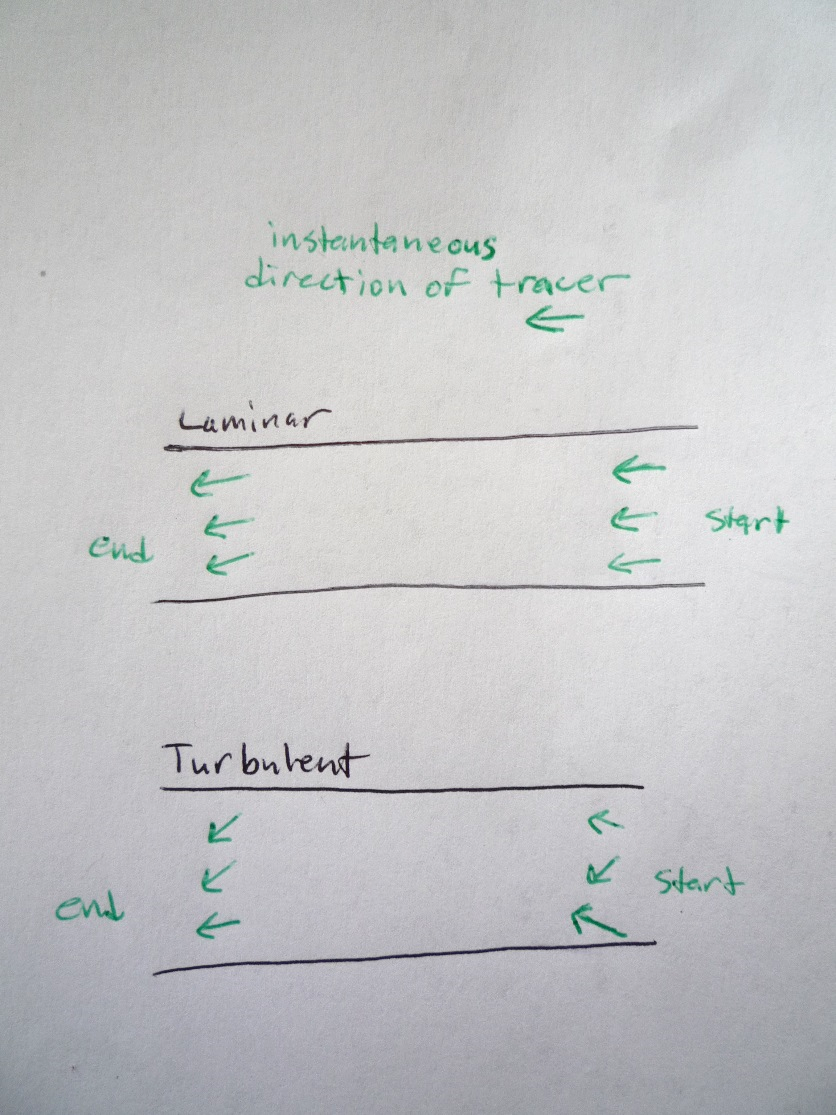
- 8. For the first video:
- a. Just after the drop hits the cream (at ~20 cm), what direction are tracers travelling? Draw 3 arrows to show the directions of 3 tracers. (call this start time)
- b. By the time the dye reaches the edge of the screen (at ~30 cm), what direction are your tracers travelling? Draw 3 arrows to show the directions of 3 tracers. (call this end time)
- c. So if you know the direction that the molecule is travelling at the start time, would you be able to predict the direction it is flowing at the end time? In other words, is knowing the starting direction sufficient information to predict the direction at a later time?
- 9. For the second video, do the same
- a. What direction is a molecule of dye travelling when it reaches ~70 cm? Draw 3 arrows to show the directions of 3 tracers. (call this start time)
- b. What direction is a molecule of dye travelling when it reaches ~90 cm? Draw 3 arrows to show the directions of 3 tracers. (call this end time)
- 10. So if you know the direction that the molecule is travelling at the start time, would you be able to predict the direction it is flowing at the end time? In other words, is knowing the starting direction sufficient information to predict the direction at a later time?
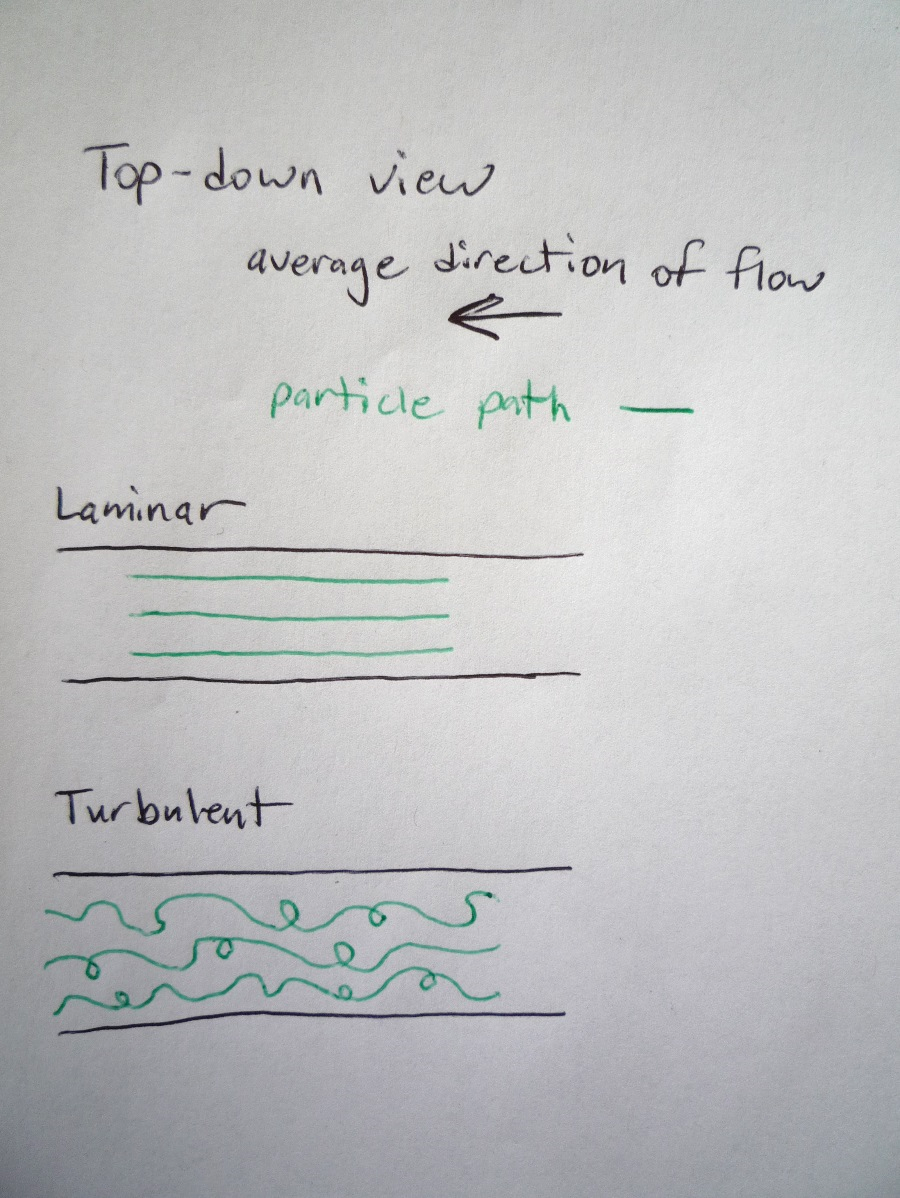
The predictable flow with straight particle paths is called “laminar” and often has a smooth appearance like the calm river. If we know the direction of flow at one time, that is sufficient information to predict the direction of flow at a later time.
The unpredictable flow with a more crazy particle path is called “turbulent” and often has an irregular or rough appearance like the river rapids. If we know the direction of flow at one time, that is sufficient information to predict the direction of flow at a later time.
Now that you know Laminar v. Turbulent, let’s discover what causes a flow to be one or the other.